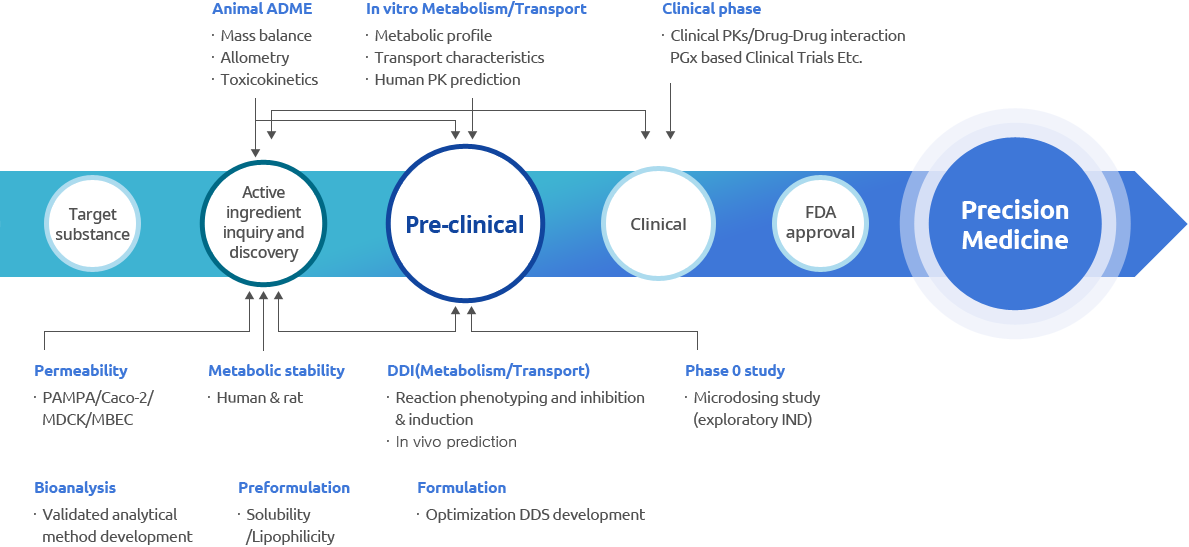
What?
Non-Clinical ADME Service
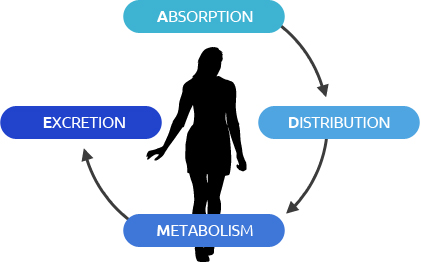
ADME, which stands for “Absorption, Distribution, Metabolism, Excretion,” is a process by which a drug reaches the target organ in vivo. The ADME service provides what is necessary to ensure drug safety and efficacy evaluation and certification.
Non-Clinical ADME Services of SPMED
-
Assessment of the potential of drug candidates to be developed as new drugs
-
Development of services according to client request
-
Non-clinical ADME test services recommended by the US FDA
-
Various networks formed with domestic and foreign researchers specializing in drug metabolism/carriers and pharmacokinetics
-
Extensive ADME-based technology related to drug metabolism and carriers
-
Participation of researchers who have performed pre-clinical and clinical projects in a number of new drug developments at home and abroad
About In Vitro ADME?
ADME, which stands for “Absorption, Distribution, Metabolism, Excretion,” is a process by which a drug reaches the target organ in vivo.
During new drug development, in vitro ADME research concerning drug metabolism and transport can reveal information on drug stability,
metabolic/delivery properties, pharmacokinetic properties (PK) and so on.
Why?
-
- Since drug metabolism and pharmacokinetics (PK) factors have been known to cause major failures in new drug development or clinical research, the success rate has been enhanced by introducing ADME evaluation studies in the early stages of drug development.
-
Experts say that the reason for reduced PK failure rates has been the introduction of a simple in vitro absorption characteristics screening test that includes Caco-2 screening.
-
At present, examining the metabolic/delivery characteristics of candidates (i.e., in vitro ADME study) in the early stages of new drug development is an important strategy for saving time and money, especially for multinational pharmaceutical companies.
-
Also, based on the recommendation of the US FDA, EMA, and PMDA, non-clinical ADME studies are being carried out in the early stages of new drug development projects.
When?
By carrying out in vitro ADME evaluation studies in the early stages of, and throughout, new drug development projects, it is possible to better understand the metabolic/delivery characteristics and pharmacokinetic (PK) characteristics of candidates, thereby reducing failures in drug development resulting from problems associated with ADME.
Non-Clinical ADME Services of SPMED